Moreno-Hernandez Lab highlights the importance of understanding nanoscale dynamic restructuring
Hydrogen has the potential to be a great alternative to fossil fuels, specially “green hydrogen” which is produced by using electricity from renewable sources (e.g., wind, solar or hydroelectric) in devices called electrolyzers which split water into hydrogen and oxygen.
Corrosion of catalysts inside electrolyzers is one of the main issues with the production of “green hydrogen” that prevents the adoption of electrolyzers at a global scale.
The Moreno-Hernandez Laboratory focuses on understanding how the structure of these catalysts affect their ability to efficiently split water (activity) and their ability to resist corrosion (stability). These studies help inform the design of better electrolyzer materials. Key techniques in the Moreno-Hernandez Laboratory are liquid-phase transmission electron microscopy (LP-TEM), which enables the observation of individual catalyst particles in action, and proton exchange membrane water electrolysis, which tests the activity and stability of catalysts at relevant operating conditions.
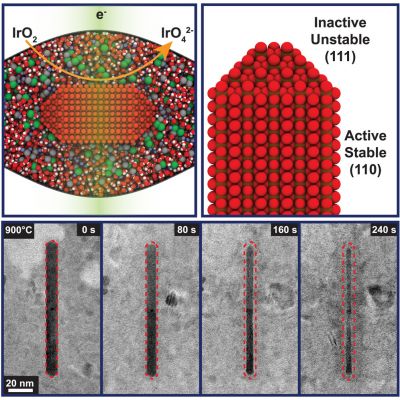
In a recent paper published in Matter on November 26th, Matteo Fratarcangeli (a second-year student in the Moreno-Hernandez Laboratory) with the help of other members of the lab, investigated the effects that a disordered structure has on the stability and activity of crystalline iridium dioxide, the leading catalyst to make oxygen in electrolyzers.
The conventional wisdom in this field is that a material which has a disordered structure will be more efficient at splitting water and more susceptible to corrosion. Using smaller versions of commercial electrolyzers, the Moreno-Hernandez Laboratory was able to replicate these observations. They observed that disordered iridium dioxide was both more active and less stable than ordered iridium dioxide.
However, observing the corrosion process at the nanoscale using LP-TEM revealed more interesting information. They observed that the tips of the nanocrystals, which are known to be inactive, corroded faster than the sides of the nanocrystals, which are know to be extremely active. Furthermore, comparing the corrosion between disordered and ordered nanocrystals, they saw that the tips corroded faster in disordered nanocrystals while the sides showed no significant difference in corrosion rate. Faster corrosion at the tips will then lead to faster corrosion of the electrolyzer as a whole.
These findings show that at the nanoscale, materials can have a stability-activity relationship that is opposite to the conventional accepted one (more disorder equals higher activity and lower stability) and that device scale observations can originate from complex nanoscale phenomena. Their study also highlighted possible ways of improving iridium-based electrolyzer materials by maximizing the exposure of active and stable disordered nanocrystal sides and minimizing the exposure or protecting the inactive and unstable nanocrystal tips. The knowledge from this study is being used to design next-generation catalysts that will decrease the costs associated with renewable energy technologies.