Overview
Academic chemistry laboratories are potentially very dangerous places. Considering the turnover of hundreds of students who must be taught to handle toxic, flammable and explosive compounds often under abnormal conditions of temperature and pressure, it is obvious that there is little margin for error or carelessness even in the undergraduate teaching laboratories. In the research laboratories many operations require pushing the handling of unknown materials or high powered equipment to extreme limits. However, long experience within the discipline of chemistry has proved that, with appropriate foresight and care, almost any kind of a chemical experiment can be carried out without an accident. The keys to safe operations in the chemistry laboratory are:
- a strong, persistent will to prevent accidents which puts safety first,
- adequate information and training to foresee and prevent accidents,
- a regular program for identifying and dealing with hazards of all kinds within the total environment of the laboratories and carefully developed plans for dealing with emergency situations when, and if, they arise.
Regarding the above:
- A strong will to work safely is such an important aspect of scientific training that it should be taken for granted of anyone with an undergraduate degree in Chemistry.
- Detailed information needed for handling hazardous operations is available in a variety of excellent books. Many of these books are listed in the bibliography at the end of this manual.
- The Duke Chemistry Department's Safety Program operates under the University and the Occupational and Environmental Safety Office (OESO). See http://www.safety.duke.edu for more information.
An additional detailed source, "Prudent Practices in the Laboratory: Handling and Disposal of Chemicals" prepared by the National Research Council can be downloaded as a free PDF from the National Academies Press. This should be made available by the group Safety Officer for ready reference. Also each research group is responsible for developing a Chemical Hygiene Plan. This plan includes but is not limited to an Introduction to the Hazard Communication Standard, the Laboratory Standard, the University Hazardous Waste Policy, A Guide to the Safe Use of Peroxide-Forming Compounds, and laboratory inventory lists of toxic substances and carcinogens. More information about Chemical Hygiene can be found at Duke's OESO website. The present safety manual is a short, ready reference to some of the most common and immediate dangers and also the rules and procedures for reacting to emergencies in this Department. It is not meant to be an exhaustive discussion of laboratory safety.
Safety Manual Sections
Click on each section header to read its contents.
Safety Contacts
Chemistry Department Safety Committee
| Name | Title | Phone | Home Phone | Room |
|---|---|---|---|---|
| Dr. Farrell Kersey | Safety Coordinator / Undergraduate Lab Manager | 660-1615 | 1225 | |
| Dr. Peter Silinski | Director of the Chemistry Shared Instrument Facility | 660-1532 | 2325 | |
| Ms. Kristen Southworth | Administrative Manager | 660-1507 | 3237 | |
| Prof. Dewey McCafferty | Chair of Safety Committee | 660-1516 | B120 LSRC | |
| Prof. Katherine Franz | Chair of the Department of Chemistry | 660-1508 | 3236 |
Duke Occupational & Environmental Safety Office - Staff Directories
https://www.safety.duke.edu/staff-directories
Elementary Safety Rules
- Keep this manual within easy access in your laboratory and be familiar with its contents.
- The safe way is the right way to do your job. Plan your work. Follow instructions. If you do not know how to do the job, ask your instructor or research director.
- Report to the Safety Coordinator all unsafe conditions, unsafe acts and "near misses" which might cause future accidents.
- Be able to use all safety devices and protective equipment provided for your use. Know the location and contents of the nearest safety station.
- Maintain good housekeeping by keeping your work area clean and orderly.
- Wear proper clothing. Avoid bringing long hair, loose sleeves, cuffs, rings, bracelets, etc. in proximity to moving machinery. Proper shoes are required in the laboratory — no bare feet or sandals.
- Horseplay in any form is dangerous and prohibited. Do not run in laboratory areas or halls.
- Do not oil, grease, or work on unprotected machinery in motion.
- All machinery and equipment under repair and adjustment shall be properly "locked out" and tagged.
- Know the evacuation procedure for your area, the location of fire exits, the location and use of fire extinguishers, and the proper method of reporting fires.
- Compressed gas cylinders should be secured firmly. Never move a cylinder unless the protective cap is screwed over the valve.
- Don't try completely new and untried experiments involving potentially dangerous chemicals without help.
- Changes to common procedures, including: "scaling-up" a reaction; a change in heat source or reaction temperature or pressure; change in solvent; etc., turn a known procedure into a high-risk procedure. Be sure to discuss all changes to known procedures with the Principal Investigator, the Safety Representative from the group, the Departmental Safety Coordinator, and/or the OESO.
- An Unattended Experiment Form (downloadable below) should identify any laboratory where an experiment is to be left unattended overnight or over a weekend. It is your responsibility to see that adequate information is supplied to protect safety personnel or firemen who may have to deal with an emergency situation in your laboratory.
- Never leave a reaction or experiment running unattended unless you have told your lab partners enough about it to deal with potential hazards while you are away. Leave an overnight form on the door if the laboratory will be unattended.
- Never carry out hazardous work alone, especially at night or over the weekends. The National Safety Council makes the following recommendations: Make sure someone is in visible or audible range to help you if something goes wrong. Regardless of the work function, there should be a check procedure established at some regular interval to determine the physical state of the person working alone. Keep aware of where your neighbors are.
- Report every accident or fire, no matter how trivial, at once to the Campus Police, 684-2444. Even if there is no injury to personnel or equipment, a report should be filed by a police officer. If the incident is trivial, give Campus Police the option of sending an officer to your lab at a mutually convenient time rather than immediately.
- Smoking is prohibited in French Family Science Center.
General Safety Policies
The safety and well being of its students, faculty, and staff come above all other considerations at Duke University. No experiment that subjects personnel to unreasonable risk is acceptable, no matter how desirable the information which might be obtained. It is the first duty of research directors, instructors, supervisors and all persons in authority to provide for safety in the environment and operations under their control. It is the Chemistry Department's policy to comply not only with legal safety standards, but to act positively, where it can, to prevent injury, ill-health, damage and loss arising from work carried out within its building. The Department seeks to encourage all its members to participate in and contribute to the establishment and observance of safe working practices. This is not only a moral duty, failure to do so can constitute legal grounds for negligence suits. A discussion of Negligence Suits in Chemistry Teaching, J. Chem. Educ. 60, 358 (1983) states, "In all cases it is the teacher who is legally responsible for the safety of his or her students. The teacher must foresee hazards to the extent that any reasonably prudent person would". An aim of this manual and the Duke Chemistry Department safety program is to provide the required information on which to base a prudent approach to safe laboratory operations.
Safety Guidelines for Undergraduate Independent Study
- Students should be assigned to work in laboratories in which graduate students and/or postdoctoral associates are also working.
- Students should receive instruction and close supervision directly from their faculty mentor, although a senior graduate student or postdoctoral associate working with the faculty member may also be involved.
- Students should not work alone, particularly at night or on weekends, on operations involving chemicals or other hazards of the type covered in the Chemistry Department Safety Manual. If work at night or on weekends is required, it should only be done with the express permission of the faculty mentor and with specific arrangements to avoid working alone.
- Supervising faculty and, if appropriate, associate supervisors, should discuss with the student the potential hazards of all experiments to be carried out, and closely supervise preparations for all new potentially hazardous operations.
- Students should agree with their supervising professors on a weekly work schedule and should make every effort to maintain this schedule.
- Students must read the French Family Science Center Safety Manual and sign a statement that they have done so, before they are allowed to begin laboratory work.
- An outline of the independent study project, including the goal(s) and as far as practical, the kinds of experiments to be carried out, methods to be used and data to be collected, as well as a proposed schedule of accomplishments, should be completed (by the student and faculty member together) before the beginning of any hands-on laboratory work. This will serve as a guide so that it is clear to student and mentor what each expects at each stage of the project and for the overall project. It would be appropriate for each to sign and keep a copy. This document should also state where the lab space for the project is and who else is to be involved in the supervision (if anyone).
- In order to comply with the above guidelines, those faculty without graduate students or postdoctoral associates or without lab space should enter into collaborations with other faculty who have students and facilities, or at least make arrangements to "borrow" appropriate lab space. If at all possible, these arrangements should be made (at least tentatively) before students are accepted for supervision. If necessary, the Coordinator of Independent Study can be called on to facilitate arrangements.
Last updated 07/25/2013
Responsibility of the Research Directors and Instructors
The first responsibility for laboratory safety lies with faculty members (Principle Investigators) in charge of the laboratories. It is their duty to evaluate the safety hazards connected with any experiment and to avoid conducting any experiment which cannot be carried out without excessive risk to personnel or property. It is also the responsibility of the faculty members to be certain that every person working in their laboratories is aware of the safety hazards and safety regulations in the laboratory. It is highly recommended that the person in charge of a laboratory have safety rules posted prominently in convenient locations for everyone to read. Research directors and teaching laboratory directors have the primary responsibility for enforcing regulations on solvent storage, waste solvent disposal, smoking, personal protective equipment, etc., and for reporting problems to the Chemistry Department Safety Committee. Research directors should recognize that graduate students and postdoctoral students coming into the Department may have enormously different backgrounds and attitudes towards good laboratory practice and that it is part of the advisor's responsibilities both in laboratory courses and as research directors to provide instruction where it is necessary either in techniques or in attitudes which are appropriate for the training of professional scientists. It is expected that research directors appoint at least one member of their research groups as a safety officer who will be responsible for maintaining safety devices, attending all monthly safety meetings and checking day-to-day laboratory safety practices. Research directors should have cards fastened to the outside of the door of each laboratory and chemical storage area under his/her supervision which states:
- The name of the faculty member or person in charge of that laboratory who should be notified in case of emergency.
- The phone number (home and business) of that person.
- Special Precautions (if necessary)
- for workers in that laboratory
- for firemen or other emergency personnel
Individual Responsibilities
Before any keys to the French Family Science Center can be issued to a new member of the chemistry department, s/he must participate in online safety training and pass the appropriate online quiz(zes). Persons engaged in the use of chemicals and apparatus inside the Department (undergraduate students, graduate student researchers, postdoctoral fellows, etc.) are responsible for protecting themselves and their neighbors. The individual student or researcher has to take the initiative in protecting himself or herself from hazards which have been explained to them, e.g. they should protect their own eyes by wearing safety glasses. Their next responsibility is to their neighbors.
University and OESO Responsibilities
See http://www.safety.duke.edu for more information on OESO Laboratory Safety.
- Proper disposal of chemical and radioactive waste http://www.safety.duke.edu/environmental-programs
- Proper maintenance and distribution of fire extinguishers and fire alarm testing and maintenance of equipment http://www.safety.duke.edu/fire-life-safety
- Inspection of emergency showers and eye wash equipment
- Ready availability of personal protective equipment
- Online safety training is required for all personnel working in a lab. Access to the training modules is gained by accessing the OESO home page and click on "Training & Reports" and then on "Online Training". You will log in using your NetID and password.
Safety Coordinator Responsibilities
- planning and presentation of Departmental safety meetings
- keeping alert to new hazards which develop in the use of chemicals and informing research directors of these hazards
- planning emergency drills
- maintaining a regular inspection procedure so that at least once each year all research and teaching laboratories have been inspected with reports submitted to the research directors
In addition, the Safety Coordinator is available to help members of the Department with individual safety problems. Potential safety problems should be discussed with the safety coordinator. All accidents or near-misses should be reported. This may help to prevent future accidents. See Duke's Occupational & Environmental Safety Office page for information on Chemical Hygiene Plans, SOP information, PHS list and MSDS information.
Housekeeping
The laboratory should be kept clean and free from clutter, by regular maintenance. At the completion of each experiment, equipment should be cleaned and properly stored. Do not let unused equipment or chemicals accumulate in the lab. Do not use the aisles of the lab or the space in front of the emergency escape panels for storage. Dispose of all hazardous wastes in accord with the procedures indicated in this manual. Reagent bottles must be properly labeled — when pouring hold the bottle with its label to your palm to protect the label. Notify your safety officer of bottles whose contents are in doubt.
Hygiene
- Wash hands often — always before eating, smoking, or leaving the laboratory. Washing should be an instinctive reaction to spillage of any chemical on the skin.
- Never eat or drink in the lab — never use lab equipment as a food or drink container.
- No food items should ever be stored or even cooled in a laboratory refrigerator. Food and beverages can become contaminated within a very short period of time to a life-threatening level by absorption of chemical vapors. Any food/beverage found in inappropriate areas will be removed without notice.
- For more information see the OESO Laboratory Safety discussion on chemical hygiene at the following URL: https://www.safety.duke.edu/laboratory-safety/chemical-hygiene
Eye Protection
Various types of eye protection listed in order of increasing effectiveness include:
- Ordinary spectacles
- Safety glasses with side shields
- Protective goggles, which can be worn over spectacles, if necessary
- Face shields
- Head shields, which protect all of the head and throat
Chemistry Department policy requires that all persons wear, at least, safety glasses (equipped with side shields), or goggles for eye protection while in the laboratory. In situations in which there is potential of a corrosive chemical being splashed into the eyes, safety glasses or goggles AND a face shield are required. In situations where there is potential for an explosion to occur, head shields are required in addition to safety glasses or goggles. Department policy on contact lenses in the laboratory is that you may wear contact lenses, but only if your eyes are protected as described in the preceding paragraph by safety glasses or goggles, with or without a face or head shield. Normal eye protection is required when you are wearing contact lenses since contact lenses provide little to no protection from chemicals in the eye. (In fact, contact lenses can complicate flooding the eye with water should a chemical get in the eye.) Safety goggles, with side shields are provided for chemistry employees (including all research students and teaching assistants) at no charge. Please see an Undergraduate Lab Manager or the Preparator. Prescription safety glasses are available (at your expense) through a local optometrist. Students who wear prescription glasses, and who do not wish to wear safety goggles (available at Bryan Center Store), must cover the costs involved in being fitted with prescription safety glasses. All undergraduates are expected to purchase and wear safety glasses at all times when they are working at their laboratory benches or in any area where hazardous activities could endanger their eyes. Teaching Assistants and faculty supervising them are expected to enforce this regulation at all times. Teaching Assistants are reminded that the safety performance of classes under their regulation is one of the criteria by which they will be evaluated by the faculty.
Foot Protection
All persons in labs must wear shoes (bare feet or sandals are not allowed) and adequate clothing to protect the skin from spilled chemicals.
Skin Protection
Always wear clothing that minimizes the amount of skin that can be exposed to potentially harmful chemicals. Never wear shorts in the lab. A lab coat or apron should be worn when working with hazardous materials. From DUKE OESO News, December 1997, Vol. 5, Number 4, pp 4-5. Of the ways chemicals can affect the human body, exposure through skin contact is one of the most significant. The skin does a wonderful job acting as a barrier to those conditions normally encountered in the environment; however, as our workplace environments use more and more chemical substances, our skin can no longer provide adequate protection on its own. Chemical substances can act on unprotected skin in three ways:
- Local Damage The action of many chemicals is limited to the skin itself. Corrosive burns, irritation, and chafing due to loss of skin oils are a few examples.
- Sensitization Sensitizer chemicals may not have any initial effect, but will cause the skin to react, during subsequent exposures, to quantities much smaller than would otherwise have any affect.
- Absorption The skin provides no barrier against some chemicals, which can penetrate freely and enter the blood stream affecting such target organs as the liver and nervous systems.
A chemical may cause damage by more than one of the above effects. Some examples include chlorinated solvents, such as ethylene dichloride, which will defat the skin causing irritation and tissue breakdown, also can permeate the skin possibly causing liver and kidney damage.
Hand Protection
From DUKE OESO News, December 1997, Vol. 5, Number 4, pp 4-5. Our hands are the body parts most likely to be exposed to chemical contact under normal situations. Even though careful technique may help an employee avoid direct contact with a chemical; the potential for exposure still demands the use of protective gloves. The department of Chemistry maintains a small list of glove compatibilities. These charts are by no means complete. If you have any questions regarding the effectiveness of a glove with a specific chemical, contact OESO or visit the following glove manufacturer links:
Selecting A Glove: What Material is Best?
"Karen E. Wetterhahn, professor of chemistry.... in the the Sciences at Dartmouth College, died June 8 at age 48 from mercury poisoning....While preparing the mercury NMR standard in a fume hood, Wetterhahn spilled one to a few drops of dimethylmercury. The compound permeated the latex gloves she was wearing and was absorbed through her skin into the bloodstream." C & EN, June 16, 1997, page 12.
In choosing a glove that will provide an adequate level of protection, it is important to keep three warnings in mind:
- There is no such thing as an "impermeable glove"
- No one glove material is a barrier to all chemicals
- For certain chemicals, there is no glove material available which will provide more than one hour of protection
All chemicals will permeate through all glove materials. This process involves absorption of the chemical at the outside surface, diffusion of the chemical through the glove, and then desorption of the chemical from the inside of the glove. Gloves are considered protective if the rate of this permeation process is slow enough that the chemical does not break through to the inside. Glove manufacturers use two measures of glove suitability:
- Permeation Rate; the amount of a chemical which is passed through a given area of glove material per unit time and
- Breakthrough Time; the elapsed time from initial contact of the chemical to the outside of the glove to the first detection of the chemical on the inside glove surface.
The objective in choosing a glove should be to seek a low permeation rate and a high breakthrough time, keeping in mind some of these factors:
- Temperature Permeation rates increase and breakthrough times decrease with increasing temperatures. You need greater protection if your chemical processes involve heat.
- Thickness Permeation is inversely proportional to thickness. Breakthrough time is directly proportional to the square of the thickness. Double gloving can quadruple the duration of protection.
- Solubility Permeation is a direct function of the solubility of the chemical in the glove material.
- Resistance To Physical Damage Do you need a glove that resists abrasions, cuts, punctures or tears? A torn glove made of the perfect material is less protective than an intact glove made of another reasonably adequate material.
- Flexibility Is the ability to feel or manipulate small objects important or can you use a thicker more protective glove?
- Heat Resistance Do you have a hot operation? As stated above, heat increases permeation rates. In addition, heat can lead to the breakdown of the glove material.
- Incompatibilities Are you using a variety of chemicals? Some gloves may be incompatible with some of the substances. A good example of this is Poly Vinyl Alcohol (PVA), a glove material with great resistance to solvents, but that dissolves in water.
Selecting a Glove: What Other Factors are Important?
There are many other factors that must be considered when selecting the glove that best suits the task. In some cases, your task may require you to choose a glove material which has a higher permeation rate, but has other qualities which makes it better for your situation. Some of these factors include:
- Disposable latex and PVC gloves have an important role in laboratories and health care settings; however they are not suitable for direct contact with aggressive or highly toxic chemicals.
- Sometimes the ideal glove is two gloves worn together, combining the advantages of both.
General Guidelines For Glove Use, Care, and Hygiene:
- It is usually not necessary to replace reusable gloves unless they become discolored or show signs of damage. If you suspect that they have been contaminated, replace them immediately - once a chemical has begun to diffuse it will continue to diffuse even when the chemical on the outside has been removed. Never reuse disposable gloves!
- Store reusable gloves away from chemicals. Even chemical vapors may cause damage.
- The use of protective gloves within the laboratory is essential in many instances. However, it is important to realize that if you are wearing gloves while handling chemicals, you must never come in contact with any item that a person not wearing gloves could. For instance, if you are entering or leaving the lab, DO NOT touch the door handle with your gloves on. While you are clearly unaffected by this action, any contaminants on your gloves will be transferred to the hand of the next person that opens the door with an ungloved hand. Likewise, remove your gloves if you are pressing elevator buttons, using a computer keyboard, using a pen that might also be used later by yourself or another person not wearing gloves, etc. Also, do not touch your face, hair, etc. while wearing protective gloves.
The intent of this article was to present an overview of the complex nature of selecting the correct glove for adequate protection. Users should consult chemical MSDS's, glove manufacturer's literature, and the OESO for assistance.
Respiratory Protection
Fume Hoods
Fume hoods provide constant respiratory protection in all laboratories in the building. Such protection is adequate for most controlled experiments. In using the hoods in the building, the following facts should be kept in mind. Additional information may be found in the OESO Laboratory Safety Manual or via the link on the OESO Laboratory Environment website labeled "OSHA Chemical Fume Hood Quick Facts".
- Lowering the sash will increase air velocity and offer greater protection from toxic fumes. Normally the sash opening should be less than 18". If the sash is opened to a height greater than 18", an alarm activated. It is important to keep the sash below the 18" mark. The OESO periodically measures the airflow in the fume hoods. They have labeled the hoods with the maximum recommend sash height. If the sash is raised above the indicated height, then the airflow will fall below the minimum acceptable level of 75 feet per minute. If you have questions about the airflow in your fume hood, contact the OESO at 684-2794.
- Placing equipment no less than six inches in the hood will also reduce the possibility of fumes escaping into the laboratory.
What does that gauge on my chemical hood mean?
The chemical fume hoods in the Department have been fitted with digital meters that provide the face velocity of airflow in feet per minute or FLO. FLO indicates the hood sash is fully closed so there is no face velocity. Makeup air is still being exhausted from the hood, but its velocity is not indicated on the gauge.These gauges allow employees to check that the hood is functioning properly (i.e., have a minimum face velocity of 75 ft/min) every time they use the chemical hood. If a hood is not functioning properly, it should not be used and employees should call maintenance. Also place a sign on the hood so that others will not use it.
- safety shower
- eyewash station
- one or two fire extinguishers
- fume hood(s)
- a first aid kit (which the research group is responsible for restocking with items purchased at the stockroom)
- a flashlight for use in case of a power failure (optional - supplied by the research group)
Emergency Respiratory Protection
There are two types of emergency equipment available for respiratory protection: air-purifying and self-contained. These should only be used by Safety Office Personnel or others who have been instructed in their use. If you have need of respiratory protection equipment, contact the OESO.
General Safety Equipment Available in Each Lab
In each lab there should be the following safety equipment: If you use any of the expendable safety items (such as a fire extinguisher), notify the OESO at 684-2794 as soon as possible so that replacements can be obtained.
Other Safety Equipment Available in French Family Science Center
Spill Kit
Adjacent to the ice machine on the first level (hallway near 1112) there are spill kits for handling common spills involving 1) Solvents, 2) Acids (not for use with HF spills) and 3) Caustics. Directions for the use of each kit are provided with each kit. If you use one of these kits, notify the Lab Preparator (Room 1214) so that the used kit will be replaced. The used materials from the kit are to be kept in your lab (in a hood) and are to be treated as a hazardous waste. It is necessary to fill out a form requesting that this waste be removed from your lab by the OESO. (See the section dealing with disposal of waste chemicals later in this manual.)
Last updated on 7/25/2013
Never carry out experiments involving a fire risk at night or over the weekend unless a colleague is nearby. Know the location of your nearest shower, fire extinguisher, fire alarm pull station, telephone and emergency exit route from your own lab. Fires and explosions are major contributors to loss of life and property in laboratories. A study of one hundred significant laboratory fires by the National Fire Protection Association provides some interesting facts: 71% of the fires originated in the laboratories; 56% of the laboratory fires originated between 6 PM and 6 AM; 67% of the fires were caused by:
- electrical equipment (wire and appliances) 21%
- misuse of flammable liquids 20%
- explosions 13%
- gas 7%
- spontaneous ignition 6%
For more information on fire safety, visit the OESO fire safety site.
Electrical Equipment
- Be careful not to spill flammable liquids around electrical equipment in use.
- Ground equipment to avoid electrical arc or spark formation from static.
- Avoid temporary wiring.
- Replace defective cords.
- Keep equipment in good working condition.
Flammable Liquids
Any liquid having a flash point below 140°F and having a vapor pressure exceeding 40 LB/sq. in. absolute at 100°F.
- Class IA: flash point below 73°F and B.P. below 100°F.
- Class IB: flash point below 73°F and BP at or above 100°F.
- Class IC: flash point at or above 73°F and BP below 100°F.
Flammable liquids should be stored in OSHA approved Safety Storage Cabinets (yellow safety cabinets).
Combustible Liquids
Any liquid having a flash point at or above 100°F.
- Class II: flash point at or above 100°F.
- Class IIIA: flash point at or above 140°F and below 200°F.
- Class IIIB: flash point at or above 200°F.
Safe Handling and Storage of Flammable and Combustible Liquids
Safety Suggestions for Handling Combustibles:
- Limit the amount of combustibles in the laboratory.
- Keep combustibles a safe distance from heat sources and stored at least 18 inches below the ceiling.
- Enforce "No Smoking" rules in applicable areas. Never use the trash can to extinguish smoking materials.
Safety Suggestions for Handling Flammable Liquids:
- Limit the quantities at any one location to those actually necessary, but not to exceed the limits specified.
- Use only approved containers, e.g., safety cans or metal drums for all transportation and handling.
- Label all containers used for liquids with the name of the material and the words: "DANGER - FLAMMABLE (or COMBUSTIBLE)" - Keep away from heat, sparks, and open flames - Keep closed when not in use.
- When pouring liquids with a low flash point from a large (e.g. 5 gal.) can, ground the can to reduce development of static charge. This is particularly important in cold, dry weather.
- Flammables should be stored in OSHA approved cabinets and must not be allowed to collect on benches, in hoods or on shelves in violation of the OSHA limits.
Maximum Quantities of Flammable and Combustible Liquids outside of Flammable Storage Cabinets:
The maximum quantity of Class I Flammables outside of the storage cabinet shall not exceed 2 gallons per 100 square feet of laboratory space. The combined maximum quantity of Class I, II and III Flammables and Combustibles outside of a storage cabinet shall not exceed 5 gallons per 100 square feet of laboratory space.
Types of Fires
Many fires are small at origin and may be extinguished by the use of portable fire extinguishers. The proper type of extinguisher for each class of fire will give the best control of the situation and avoid compounding the problem. The classification of fires given here is based on the type of material being consumed.
Class A Fires
Fires in ordinary combustible materials, such as wood, cloth, paper, rubber and many plastics. Almost any fire extinguisher is effective on a CLASS A fire, but water is the best extinguishing agent.
Class B Fires
Fires in flammable liquids, gases, oil, paint and greases. Foam, dry chemical or CO2 extinguishers are the most effective on CLASS B fires. Do not use water.
Class C Fires
Fires which involve energized electrical equipment where the electrical non-conductivity of the extinguishing agent is of importance. Use Carbon Dioxide or Dry Chemical extinguishers. Do not use water.
Class D Fires
Fires in combustible metals, such as magnesium, titanium, zirconium, sodium, lithium, zinc and potassium. Use metal fire extinguishing agent at safety stations or sand, or vermiculite.
Types of Fire Extinguishers
There are three main types of fire extinguishing agents in the building, the carbon dioxide extinguisher, the dry chemical extinguisher, and the metal fire extinguishing agent. Every research laboratory and almost all teaching laboratories are equipped with carbon dioxide extinguishers. The CLASS D extinguishing agent must be purchased by individual labs whose research requires this type of extinguisher.
Carbon Dioxide (CO2) Extinguishers
These extinguishers are intended primarily for use on CLASS B and CLASS C fires. They have a limited range; thus, initial application must start reasonably close to the fire.
On all fires the discharge should be directed at the base of the flames using care not to spread the fire by blasting burning materials around the area. CO2 discharge should be applied to the burned surface even after the flames are extinguished, to allow added time for cooling and to prevent possible re-flash.
On flammable liquid fires, best results are obtained when the discharge from the fire extinguisher is employed to sweep the flame off the burning surface, applying the discharge first at the near edge of the fire and gradually progressing forward, moving the discharge horn from side to side.
Dry Chemical (ABC) Extinguishers
Dry chemical extinguishers are intended for use on CLASS A, CLASS B, and CLASS C fires.
The discharge should be directed at the base of the flames. Best results are obtained by attacking the near edge of the fire and progressing forward, moving the nozzle rapidly with a side-to-side sweeping motion with care not to blast flaming liquid around the area.
Discharge should be continued after flames are extinguished to prevent possible re-flash.
For CLASS A fires the discharge should be continued intermittently to coat flowing areas of CLASS A materials.
Dry Powder Extinguishing Agent (D)
Dry powder extinguishing agent is intended primarily for use on metal fires.
The application of the agent should be of sufficient depth to adequately cover the fire area and provide a smothering blanket. Additional applications may be necessary to cover any hot spots which develop. Care should be taken to avoid scattering the burning metal.
Where the burning metal is on a combustible surface, the fire should be covered with powder, then a two inch layer of powder spread out nearby and the burning metal moved onto this layer, with more powder added as needed.
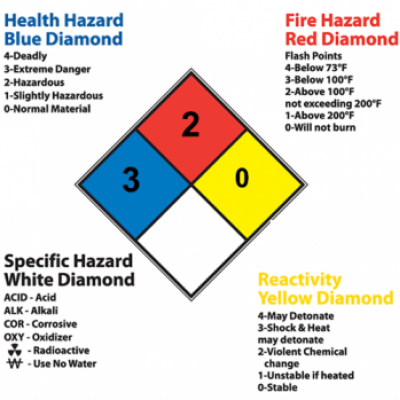
NFPA Notation for Rating Chemical Hazards
Information concerning the hazards associated with a chemical can be obtained quickly from a notation developed by the National Fire Protection Association (NFPA). OESO has documented this classification system at the following link: https://www.safety.duke.edu/sites/default/files/Section_6_FireSafety.pdf. This notation uses a diamond shaped symbol which is subdivided into four square segments. The left segment indicates health hazard which includes both contact with the chemical and inhaling the fumes from the chemical, the top segment indicates flammability, the right segment indicates instability, and the bottom segment is reserved for special warnings. A number is used in the first three segments to indicate the degree of hazard with 4 implying severe hazard, 3 for serious hazard, 2 for moderate hazard, 1 for slight hazard, and 0 for minimal hazard. If one of these first three segments is left blank or contains a dash; it does not mean that it is safe, but rather means that it has not yet been included in the NFPA listing. In the fourth segment; the notation, W with a line drawn through it is used to warn of a possible violent reaction with water, and the notation, OXY, is used to warn of a strong oxidizing agent which may react explosively with combustible materials. See the sample given below.
| 4 = Severe Hazard 3 = Serious Hazard 2 = Moderate Hazard 1 = Slight Hazard 0 = Minimal Hazard |
It should be remembered that the ratings given in the NFPA system apply to the pure chemical and generally represent the "worst case scenario". Aqueous solutions of the chemical are usually less hazardous than the pure chemical. In general, the more dilute the solution, the less hazardous it becomes. Even so, as a general policy you should avoid contact with and inhaling the vapors of all pure chemicals and their solutions.
Labeling
There are few greater potential hazards around the laboratory than that of unmarked or improperly labeled chemicals. All chemicals must have complete identification securely fastened to the container. Chemicals of unknown stability and those which deteriorate with age shall have a preparation date clearly indicated on the label. Disposal of unlabeled bottles is dangerous and therefore very expensive and tightly regulated by law. Research Directors will be required to pay the costs for removal of unlabeled bottles in their areas if their students have been responsible for producing them. The purpose of proper labels is multifold:
- They are required.
- They indicate the source, supplier, or manufacturer of the chemicals.
- They indicate the age of the chemical.
- They warn about the possible hazards.
Each research group should have some type of actively updated inventory of the chemicals in their laboratories. Most chemistry laboratories use an excel spreadsheet to maintain an active chemical inventory.
Laboratory Cleanliness
- The laboratory should be kept clean and free from clutter, by regular maintenance. Do not let unused equipment or chemicals accumulate in the lab.
- Reagent bottles must be properly labeled - when pouring hold the bottle with its label to your palm to protect the label. Notify your safety officer of bottles whose contents are in doubt.
- Never eat or drink in the lab - never use lab equipment as a food or drink container.
Transport of Chemicals
- Never transport open containers of chemicals through the hallways, stairs or in the elevator. All chemicals, with the exception of those contained in sealed metal cans, are to be transported in rubber buckets or chemical transport carts (with special dividers to hold glass bottles). Stockroom personnel have been instructed not to allow any chemicals, except those in sealed metal can, to be removed from the stockroom unless they are transported in a rubber bucket or a chemical transport cart. Research groups which must transport large amounts of chemicals have purchased one or more rubber buckets and keep these available in their labs. Persons who transport chemicals less frequently may borrow a rubber bucket to transport chemicals from the stockroom to their labs. Borrowed buckets must be returned to the stockroom or left in the corridor for someone else to use.
- Do not use a cart without side rails for transporting reagents in glass bottles even when the bottles are in rubber buckets since the buckets may fall from the cart and the bottles may break.
- Gas cylinders must be transported in approved carts with the cylinders secured by straps and capped.
Rules for Chemical Storage
- Avoid overhead storage of hazardous liquids and dangerous solids.
- Use flammable or corrosive cabinets for most storage.
- Refrigerate flammables only in approved flammable storage refrigerators.
- Maximum separation of reactive chemicals minimizes risk. Therefore, don't store chemicals in alphabetical order--store by category. Do not store mutually-reactive chemicals near each other - e.g. sodium near the sink or in a sprinkled storage area, acids near bases, organometallics near alcohols. See this manual and "Prudent Practices in the Laboratory: Handling and Disposal of Chemicals" p. 54 for tables of chemical incompatibilities. Contact OESO or the Departmental Safety Coordinator for a suggested shelf pattern for storage of chemicals.
- Date ethers and other peroxide-forming compounds upon arrival and follow directions for storage, testing and disposal given in this manual.
- Respiratory assailants and "stench" compounds should be stored in a properly vented storage cabinet.
- Store cleanup kits close to storage areas.
General Chemical Hazards
All chemicals should be regarded as potentially dangerous. Before working with chemicals with which you are unfamiliar, consult the Particularly Hazardous Substances List (Table IV of this manual) and the other tables at the end of this manual or another book (see Bibliography). You should become aware of the following possible hazards: toxicity (often quoted in terms of the threshold limit value, TLV, given as PPM of air by volume, which should not be exceeded); PEL (OSHA Permissible Exposure Limit); flammability; ignition temperature; and carcinogenic properties; and you should also know the recommended method of disposal for the chemical.
- Gases. Other than sudden release of pressure risks, toxicity, due to buildup of high concentrations in the air, is the most general hazard. Toxicity and recommended maximum cylinder size for some common gases are listed in Table I.
- Other gases and volatile liquids. Explosive and toxic properties of common chemicals are listed in Table II.
- The Duke Particularly Hazardous Substance (PHS) List can be accessed through the following link: https://www.safety.duke.edu/laboratory-safety/chemical-hygiene/particularly-hazardous-substances.
Selected Types of Hazardous Substances
- Organic peroxides. These are among the most hazardous chemicals normally handled in the laboratory, being explosive and extremely sensitive to shock and other forms of accidental ignition. Take advice or consult the bibliography before using peroxides. Although some types of peroxides can be handled with relative safety, an insidious and treacherous hazard concerning peroxides is their formation from certain classes of compounds after exposure to the air. Specific examples are: 1,4-dioxane, ethyl ether, isopropyl ether, THF, tetralin, cyclohexene, decalin. See Dr. Woerner in room 220 for a complete list of peroxide forming chemicals.
- Ethyl ether and isopropyl ether. Containers should be labeled with the date they are purchased and should be tested for peroxides or disposed of within three months after opening. Ethers should be stored in dark bottles (to reduce photolytic oxidation), refrigerated, with the date of purchase on the label. If the ether has stood for over three months it should be tested for peroxides by shaking several ml. with an equal volume of 2% aqueous KI solution and a few drops of dilute aqueous HCl or H2SO4. Development of a brown color, or purple-black in the presence of starch, indicates the presence of peroxides. Alternatively, peroxide test strips can be obtained from the OESO. To remove peroxides, shake with a concentrated solution of ferrous sulfate, or sodium sulfite. Chromatography through a column of alumina is also effective. For additional information, see the section titled "A Guide to the Safe Use of Peroxide-Forming Compounds", in the University Chemical Hygiene Plan, or contact the OESO (9-684-2794). More detailed information and procedures can be found in ""Prudent Practices in the Laboratory: Handling and Disposal of Chemicals" p. 162. Each research group should have a copy of this valuable book. In checking for peroxides and destroying them, the major responsibility for keeping track of the age of ether samples, detecting peroxides and destroying them is yours. Only particularly old and dangerous looking ether cans and bottles should be made the concern of the OESO. Never move an old bottle of the above ethers which has developed white crystals at the bottom. These may be peroxide crystals. Call the OESO and warn others to stay away from the bottle until it can be removed safely. It may detonate when touched! Never use ether for an extraction until it has been tested for peroxides. It may explode during subsequent distillation. Although lithium aluminum hydride destroys peroxides and removes excess water from ethers, it is a dangerous drying agent. NEVER USE LiAlH4 TO DRY ETHERS. Use benzophenone and sodium as recommended below. A superior alternative to the use of LiAlH4 for drying ethers and other solvents is a mixture of sodium and benzophenone. This may be prepared by adding about 5 g of chunk sodium to 10 g of benzophenone in 2 l. of the solvent to be dried. This should be done under nitrogen since the mixture reacts with oxygen. A purple solution results, from which the dry solvent is distilled. Appearance of a brown or greenish yellow color indicates the depletion of the drying agent. This is a safe way of removing water, oxygen and peroxides, while leaving no dangerous residue for disposal. For a useful reference to articles about drying agents and their abilities see D. R. Burfield and R. H. Smithers, J. Org. Chem., 43, 3966 (1978).
- Cyanides and nitriles. Store away from acids. Because of the extreme toxicity of cyanides and nitriles, ampoules of amyl nitrite should be kept in laboratories where these chemicals are used. In an emergency, amyl nitrite should be held under the affected person's nose for about 15 seconds and then at intervals until help arrives.
- Organic solvents. Among the more common solvents, benzene (TLV 10 PPM; PEL 1 PPM) and carbon tetrachloride) (TLV 5 PPM; PEL 2 PPM) are particularly hazardous; the latter is readily absorbed through intact skin to produce chronic damage to the liver and kidneys.
- Perchloric acid and perchlorates. All perchlorates should be considered as very explosive, especially on contact with organic materials. Many, particularly all metal amine complexes, and perchlorates, are extremely shock sensitive, powerful explosives. Periodates and chlorates are similar hazards.
- Mercury. Mercury should be stored in polyethylene containers - not in glass bottles, because of the risk of breakage. Spilled mercury should be, immediately and painstakingly, cleaned up preferably by OESO personnel, or by using a trapped vacuum line. Materials for cleaning up small mercury spills are available in the Gross Chemistry Stockroom (room 206A). Independent of who is to clean up the spill, contact the OESO, 9-684-2794. Recovered mercury should be placed in a closed container and tagged for hazardous waste pickup. Contact the OESO to clean up any mercury you cannot recover.
- Alkylating agents. All powerful methylating agents are extremely toxic. The toxicity of iodomethane, dimethyl sulfate and diazomethane are well known. When these reagents are of inadequate reactivity, methyl fluorosulfate, methyl trifluoromethanesulfonate (triflate) and trimethyloxonium salts are frequently used. Methyl triflate has almost identical volatility to methyl fluorosulfate, is slightly more reactive but more expensive. Trimethyloxonium salts are more reactive in most cases, and may offer a safety advantage because of their low-volatility. Being hygroscopic solids of low solubility, they are more difficult to handle, and this may introduce its own safety penalty.
- We conclude that the alkylation of unreactive substance using, of necessity, powerful alkylating agents, is probably an inherently hazardous chemical operation and should be approached accordingly.
Specific Hazards of Selected Chemicals
It would be impossible to list all of the possible chemical hazards which might be encountered in laboratories. A few of the most commonly used hazardous materials are listed below.
- Nitric Acid. In addition to its corrosive properties, and the highly toxic properties of its oxides, nitric acid is a powerful oxidizing agent and forms flammable and explosive compounds with many materials. Paper, which has been used to wipe up nitric acid spills, can ignite spontaneously when dry and should not be thrown into a waste basket until first rinsed with water and neutralized. Highly flammable materials such as ethers, exposed to nitric acid may spontaneously ignite. A mixture of nitric acid and acetone has been known to explode upon standing. Storage of nitric acid should always be away from combustible materials including organic acids such as acetic acid.
- Perchloric Acid. This acid forms highly explosive and unstable compounds with many combustible materials and even with metals. Wood or asphalt tile on which perchloric acid has been spilled may spontaneously ignite. Perchloric Acid should be used with extreme caution and only in a fume hood specifically designed for this use. Perchloric and sulfuric acid, when mixed, produce anhydrous perchloric acid which will ignite any organic matter (e.g., wood) and/or explode spontaneously. Explosive crystals may form in perchloric acid bottles stored over long periods. For this reason, bottles should not be stored for more than one year.
- Picric Acid (2,4,6-trinitrophenol). This acid may form explosive compounds with many combustible materials. It may also lose water and become unstable after extended periods of storage. It should be stored wet, and, away from combustible materials and should not be kept for extended periods.
- Hydrofluoric Acid. This acid is extremely corrosive, even attacking glass, and unlike the other halogen acids, is also extremely toxic. It is volatile and will attack skin and eyes. Burns from hydrofluoric acid heal slowly and with great difficulty. There is a chance of nerve damage resulting from all HF exposure. It forms toxic fumes in contact with metals or ammonia. It should be handled only in an adequate fume hood while wearing appropriate personal protective equipment. Inhalation can be fatal. Call the ambulance at once if taken into the lungs.
- Mercury. The vapor pressure of mercury at room temperature is about 1 micron which is sufficient to produce a concentration which is several times the allowable concentration for continuous exposure. Although this concentration is not likely to occur with small spills in a well ventilated laboratory, every effort should be made to avoid mercury spills and to clean up spills which do occur. Care should be taken not to heat mercury in open vessels or to heat equipment contaminated with mercury. Mercury or mercury compounds should never be disposed of by sewer. Mercury vapors from thermometers which have broken in hot oil baths and ovens are a common hazard. To be safe, whenever spills occur, the OESO should be notified immediately.
- Lead. Lead may give off toxic vapors when heated. Lead compounds in the form of finely divided powders may be carried by air currents. Lead and lead compounds, like and heavy metal, should not be disposed of by sewer. People using lead and lead compounds are required by OSHA to have special training. Contact the Safety Coordinator before beginning to schedule such training.
- Phosphorus (Yellow). Yellow phosphorus is stored under water. It may ignite spontaneously if allowed to dry. It is extremely toxic if ingested. Gloves should always be worn when handling phosphorus. Care should be taken that the water level in the storage jar not be allowed to go below the level of the phosphorus. Material contaminated with phosphorus should be handled with great care to avoid fire hazard or exposure of personnel.
- Ether. In addition to its highly flammable properties, it may form explosive and unstable peroxides if stored over long periods. The peroxides may explode from shock or even from the friction of unscrewing the bottle cap. Tetrahydrofuran and isopropyl ether as well as the more widely used diethyl ether exhibit this dangerous property. Ether should be ordered in minimum quantities and should not be stored over long periods. Containers with visible peroxide crystals should be handled and disposed of with extreme caution by the Environmental Safety Department. Do not ever disturb such a bottle. See pages 20-21 regarding detection and removal of peroxides.
- Carbon Disulfide. Is the most highly flammable and explosive of all the common solvents. Its vapor can be ignited by contact with an ordinary light bulb or steam radiator. It is toxic, and major residual injury may result from overexposure in spite of prompt treatment. Carbon Disulfide should be handled only with adequate ventilation and protective clothing to prevent contact with the skin or eyes. Because of its low ignition temperature the danger of fire or explosion is high.
- Active Metals. (Sodium and potassium being the most common) - are stored under oil. They react violently with water and may ignite spontaneously if exposed to the moisture in air. Toxic fumes are given off during combustion. Protective clothing should be worn while handling these materials. On long term standing even when stored under oil, potassium forms a superoxide. The dry superoxide is very unstable and can explode if subjected to any form of shock.
- Oxidizing Agents and Peroxides. These materials should be obtained and stored in minimum quantities. Deterioration may occur after long storage causing an explosion hazard. Explosion hazard can be minimized by treatment with ferrous sulfate. These materials should never be stored in close proximity to flammable materials. Phosphorus pentoxide if moistened with water can easily ignite paper, for instance.
- Organic Phosphates. These compounds are related to the nerve gases developed during World War II. They are generally used as pesticides. They should be used with caution. Disposal should not be made in trash receptacles or sewers.
Chemical Spill Clean-up
Our general "rule of thumb" for dealing with spills is as follows:
- If you spill more than one liter of any chemical, or less than one liter of a moderately or highly hazardous chemical, or you are uncomfortable cleaning up the spill, call 911 from a "land line" phone or 919-684-2444 from a cell phone and report the spill to Campus Police. They will respond to the call and will contact the OESO who will send a spill cleanup team to do the cleanup.
- If you spill less than a liter of a chemical that is not very hazardous (refer to the MSDS for hazard information), and you feel comfortable cleaning it up, find out how to clean it up (also found in the MSDS for the chemical) and do so, see section on spill kits below.
- Spill Kit. Adjacent to the ice machine on the first level (hallway near 1112) and located in each research lab are spill kits for handling common spills involving 1) Solvents, 2) Acids (not for use with HF spills) and 3) Bases. Directions for the use of each kit are provided with each kit. If you use one of the kits at the ice machine near 1112, notify the Lab Preparator (x1517, Room 1214) so that the used kit will be replaced. If you use a kit located in a research lab, notify the Manager of Chemistry to obtain a replacement. The used materials from the kit are to be kept in your lab (in a hood) and are to be treated as a hazardous waste. It is necessary to fill out a form requesting that this waste be removed from your lab by the OESO. (See the section dealing with disposal of waste chemicals later in this manual.)
- If the spill involves some chemical not covered by the three spill kits, consult OESO (684-2794).
For more information check out OESO's Laboratory Safety Manual, Section 3.
Last updated on 7/25/2013
Compressed Gas Cylinders
There is no central storage of compressed gas cylinders in French Science Center. Vendors deliver and remove cylinders regularly from individual labs. Cylinders of compressed gases, including "unreactive" gases such as nitrogen and argon, should be treated as potential rockets. When a cylinder is delivered to your lab, keep the valve protection cap on until the cylinder has been secured against a wall or bench or placed in a cylinder stand and is ready to be used. Cylinders should not be stored near sources of heat e.g. ovens, sunlight. In the laboratory, gas cylinders of all sizes must be supported by straps, chains or a suitable stand attached more than halfway up the tank to prevent them from falling over. Never drop cylinders or permit them to strike each other violently. If the valve breaks off a cylinder, the rapid release of the compressed gas may cause the cylinder to start moving rapidly and dangerously. It is the responsibility of the user to install and remove the regulating valves. Be sure to use the appropriate regulator for each gas cylinder; note that different regulators have different couplings. For information about the proper use of the valves and regulators, contact OESO. Regulating valves are not to be used as shut off devices. Never tamper with safety devices in valves of cylinders. Never oil cylinder regulators or valves since this could result in a fire or an explosion. If the valve is stuck and will not close, empty the tank and request that the vendor remove it from your lab. When not in use, cylinder valves should be closed tightly and stem protector caps secured. All tanks should have a small white label on the top front of the gas cylinder indicating the present status of the tank. Indicate the status as: FULL, IN USE or EMPTY. When the cylinder is empty (<15 lbs residual pressure), request that the vendor remove/replace the cylinder. If you need to move a large cylinder more than a few feet, secure the protector cap, place the cylinder on a gas cylinder cart, and strap the cylinder securely in place. A cart may be obtained from outside the stockroom (1126) and should be returned there after use. A more detailed discussion of using compressed gases is available at http://www.airgas.com/content/details.aspx?id=7000000000010.
Liquid Nitrogen, Dry Ice
Liquid nitrogen is available from the storage closet next to room 1238 and dry ice is available from the large blue chest located inside the building near the door to the loading dock. The first time you must obtain either of these substances, ask an experienced member of your research group to show you the proper procedures for handling them. Although liquid nitrogen is the colder, dry ice has the larger heat capacity; so both can cause severe "burns". Wear appropriate thermal protecting gloves whenever handling dry ice or liquid nitrogen; and take great care when pouring liquid nitrogen or when immersing objects at room temperature in liquid nitrogen — eye protection, of course, must be worn. Bare glass Dewar flasks used for cold traps should be wrapped with medical adhesive tape or glass fiber tape, to prevent flying glass in case the Dewar implodes. Dry Ice Baths do not use acetone or ether as the liquid, since they are too volatile and flammable. Suggested alternatives are trichloroethylene or isopropanol (flammable).
Cryogens
- Extremely cold liquid and exhaust gases - USE GREAT CAUTION. Effluent lines may be at dangerously low temperatures. Do not use cloth gloves, as the liquid can saturate the material, resulting in more extensive cold damage.
- Make sure that storage Dewars have safety necks. Periodic inspections (at least once a day) should be made to insure that no air or ice plugs exist in the neck opening.
- When a 175 liter liquid nitrogen tank is depleted, close the pressure buildup valve (the valve with the metal tag marked "PB"). If the gauge shows a pressure of more than 50 lbs/sq. in, open the vent valve until the gauge reads less than 50 LB/sq. in. Follow the directions posted in the storage closet to inform the appropriate administrative staff of the empty tank to facilitate a refill request.
- Condensed liquid air (such as may accumulate in a vacuum line cold trap cooled by liquid nitrogen) will be enriched with liquid oxygen and an explosive condition may result; surfaces which may condense liquid oxygen (air) must be clean from grease and oil.
- On equipment using liquid helium, make sure that relief valves (pop-offs) are installed and that they are large enough to vent a sudden vaporization of all the liquid should the insulating vacuum fail. Note that sudden vaporization of liquid cryogens can result in an explosion due to sudden pressure changes.
- Use liquid cryogens (helium and nitrogen) in a well-ventilated area — these gases do not support life. If these liquids are to be used long term on a regular basis, an oxygen monitor should be installed in the lab.
Electrical Equipment
- The Chemistry Building uses several different electrical supplies: 110V, 220V, etc. The wall outlet of each requires a differently-shaped plug, so there should be no danger of accidentally plugging into the wrong supply.
- All electrical equipment should be grounded properly through a 3-pronged plug (consult the electrical shop if a floating ground is required). In general, other metal equipment, such as vacuum racks, should also be grounded. Protection from static electricity: ensure that metal tanks and containers are grounded (e.g. to the metal water pipes) while pouring solvents, to prevent fire or explosion from static sparks.
- Electrical equipment which is run overnight should be fused adequately and protected against damage in the event of a power failure.
- High voltage equipment (over 400V) should be clearly labeled; all high voltage leads should be caged or completely insulated.
- Check wiring to equipment periodically — chemicals corrode insulation. Avoid long cables along the floor of the lab. Electrical connections must be made by using terminal strips, not by twisting two pieces of flex together. Particular attention should be given to Variac, heating bath and magnetic stirrer power cords. Fraying of the wire on these appliances is particularly hazardous, due to their use near flammables and water. Equipment with bad cords should be repaired by a professional.
- Do not alter manufactured equipment. Disconnect electrical equipment before attempting any repairs.
If in doubt, consult the building manager (room 1133, 919-971-2517).
Procedures in Case of Electrical Power Failure
Total power outages are not expected to occur in French Science Center since there is a backup generator in operation. It would still be prudent to consider potential hazards or difficulties that would result from power failure and we recommend that you ask yourself what will happen in your laboratory:
- When the power goes off.
- When the power goes back on after a period of several hours.
- When the power goes on after several days (if there is an extended power outage).
We note the following as sources of hazard in the event of a power failure:
- All hoods will stop, until the backup generator starts. You should be prepared to deal immediately with toxic or inflammable gases.
- All pumps will stop. Vacuum lines are apt to flood with pump oil. Liquefied gases in vacuum lines may be a hazard as the supply of Dry Ice runs out.
- Refrigerators and freezers and cold rooms will warm up after a few hours. Explosive vapors may accumulate, and detonate when the power goes on. Although the thermostats in older refrigerators in research labs have been modified to reduce the potential for explosion, the compressors are not sealed and are a potential ignition source. It is therefore important to keep the contents of the refrigerator cold by keeping a block of Dry Ice in the freezer compartment for the duration of the outage.
- Power surges or sparks from switches may be a hazard for delicate equipment when the power goes on.
- The elevator will continue to operate on backup generator power.
- Assuming that the generator operates correctly, all lights except one safety light in each lab and safety lights in each corridor and stairwell will go out. If the generator fails, all lights will go out. You should bring a flashlight to work and keep it available in your working area.
- If a power failure appears to be extended you should go home and stay there after leaving your laboratory in safe condition. It will not be practical to do research anyway!
Overnight and Unattended Operation of Equipment
Do not run equipment or experiments overnight, unless it is absolutely necessary. If it is necessary, discuss with your colleagues the best and safest way to set up the equipment. Some suggestions follow.
- Where continuous water cooling is required, Tygon or metal tubing should be used and connected to faucets and apparatus by clamps or wired on. Screw-on high pressure clamps and hairpin type clamps which make it easier to clamp and unclamp hoses on condensers for overnight reactions are available in the stockroom. Water cooled equipment or hazardous reactions should be powered through an I2R water-controlled switch which will turn off the equipment if water flow drops below a safe level.
- Electrical equipment, e.g. vacuum pumps, must be fused adequately and protected against a power failure.
- Distillations and chemical reactions should involve small quantities of reagents only and be set up in a fume hood.
Whenever equipment is run unattended overnight, an Unattended Experiment Form must be completed with the name and phone number of the research worker concerned and action to be taken in emergency. Use full chemical names and not chemical symbols. This form should be posted prominently on the door of the lab where the experiment is taking place.
Refrigerators
(see also Power Failure section above) A directive from the City of Durham Fire Marshal requires that all refrigerators, freezers or coolers utilized in laboratories where chemicals are used be prominently labeled to indicate whether they are or are not suitable for storing flammable liquids. Class I flammable liquids are defined as "any liquid having a flash point below 100 degrees F and having a vapor pressure not exceeding 40 PSI absolute at 100 degrees F". Class one liquids are subdivided as follows:
- Class I‹A Those liquids having a flash point below 73 degrees F and a boiling point at or below 100 degrees F.
- Class I‹B Those liquids having a flash point below 73 degrees F and a boiling point above 100 degrees F.
- Class I‹C Those liquids having a flash point at or above 73 degrees F and below 100 degrees F.
Refrigerators utilized for storage of chemicals in laboratories generally fall within the following three types:
- Refrigerators designed to store flammable liquids with all electrical equipment that meets Class I, Division I requirements.
- Refrigerators that have been modified by a licensed electrician that meet the class I, Division I requirements.
- Residential-type refrigerators that cannot be utilized to store flammable liquids, but are used for storage of other chemicals.
- Type 1 & 2 refrigerators will require a blue and white label affixed to the refrigerator which states: "NOTICE ‹ This refrigerator is approved for the storage of flammable materials. No food or drinks may be stored in this refrigerator."
- Type 3 refrigerators will require a red and white label affixed to the refrigerator which states: "WARNING ‹ This refrigerator is not approved for the storage of flammable materials. No food or drinks may be stored in this refrigerator."
Appropriate labels for your refrigerators may be obtained from the Fire Safety Division of the OESO, 684-5609.
Vacuum Pumps
All vacuum pumps should be equipped with V-belt guards to protect the operator from possibly being caught in the belt. If any pumps are found to have this guard missing, they should be taken out of operation and sent to a repair shop for installation of proper guarding.
Radiation Safety
The safety aspects of ionizing radiation, including radioactivity and x-ray sources, fall within the general supervisory and advisory responsibilities of the University's Radiation Safety Officer who reports directly to the Chancellor. The Radiation Safety division of the OESO has the responsibility of monitoring all radiation activities on the campus. The director of any research group who contemplates using radioactivity must apply to the Radiation Safety Office to become an Authorized User under the University's license from the Nuclear Regulatory Commission. While the Radiation Safety Office surveys laboratories and monitors personnel exposures, the responsibility for safe practices in any lab rests solely with the research director. It is his responsibility to see that each member of the group has been trained in the safe use of radioisotopes. This need for explicit training is unique to radioisotope work. Injunctions to "be careful" are not enough. The Radiation Safety Office will present a training course several times a year. Two other essential prerequisites for any laboratory using radioactivity are a sensitive survey meter and a carefully delineated procedure for decontaminating glassware and equipment.
Lasers
The Duke University Laser Safety Program is based on the American National Standards Institute (ANSI) document Z136.1-1993, American National Standard for The Safe Use of Lasers. The Program and its requirements will be described in the Duke Laser Safety Policy Manual and Procedures Manual. All laboratories in which lasers are used must contact the Radiation Safety Office (RSO) to register their laser system and schedule an initial laser site visit. The phone number for the RSO is 684-2194. A laser registration form is also available online.
Alignment Guidelines for Class 3B & 4 Lasers
- Allow only trained personnel to be present during alignment.
- Minimize the number of personnel present during alignment.
- Assure that all personnel present wear appropriate laser eye protection, i.e., correct wavelength and optical density.
- Avoid using beam paths that are at eye level while sitting or standing.
- Where feasible, use low power (class 2 or 3a) visible lasers to simulate the path of high power and/or invisible lasers.
- Where feasible, terminate laser beams and specular reflections on diffuse reflecting beam blocks.
- Use phosphor cards, UV/IR viewers, video cameras, or other beam display devices to locate invisible beams.
- Locate any specular reflections of the beam and block them as near their source as possible.
- Whenever possible, reduce all high power laser beams to the minimum possible power.
- Use beam shutters to block high power beams any time they are not actually needed.
- Avoid directing laser beams toward doorways, windows, or other viewing portals.
Smoking in French Family Center
Smoking is prohibited in French Family Science Center.
Alcohol and Medications
People who are under the influence of alcohol or medications that impair an individual should never conduct experiments.
Drugs
Duke University prohibits its members of its community, both individuals and groups, from manufacturing, selling, delivering, possessing, using, or being under the influence of a controlled substance without legal authorization. A controlled substance includes any drug, substance or immediate precursor covered under the North Carolina Controlled Substances Act, including but not limited to opiates, barbiturates, amphetamines, marijuana, and hallucinogens.
The possession of drug paraphernalia is also prohibited under North Carolina state law and university policy. Drug paraphernalia includes all equipment, products and material of any kind that are used to facilitate, or intended or designed to facilitate, violations of the North Carolina Controlled Substances Act.
In addition to disciplinary action, the conduct officer, or designee, may require a student to take a leave of absence, and return to campus may be conditional upon proof of completion of a substance abuse treatment program.
Last updated: 08/04/2011. Policy owner: Dean of Students.
Planning Experiments
It is expected that time and care will be taken by all occupants of French Family Science Center in PLANNING EXPERIMENTS. [The following material is taken from a safety booklet prepared by the Mallinckrodt Laboratory Safety Committee. This booklet is no longer available.] In order to foresee and avoid some of the booby-traps in a laboratory experiment:
- List all possible reactions, including side-reactions, before beginning.
- Think through all reactants, intermediates, and products in terms of flammability, toxicity, and reactivity hazards.
- Follow recognized safe practice procedures concerning protective equipment, housekeeping, the handling of hazardous chemicals and of equipment, as outlined earlier.
- In an unknown reaction, always start with small quantities of material and carefully observe reaction characteristics, such as temperature, color, viscosity, and physical state.
- Obtain safety information about reactants and possible products from Material Safety Data Sheets (MSDS). If the safety information is not available elsewhere, it may by obtained from some outside laboratories that offer a testing service for the evaluation of explosion hazards, etc.
- If possible, determine from thermodynamic and kinetic considerations, the total quantity of, and the rate of evolution of heat and gases to be released during the reaction.
- Provide adequate cooling, ventilation, pressure relief, and gas purging. Isolate the reaction vessel, if possible, and make frequent inspections of equipment during reaction.
- Do not leave a hazardous system unattended.
For each reactant, intermediate, or product, ask:
- What is its flash point, flammability range, auto-ignition point, vapor pressure and vapor density?
- Does it decompose and if so, how rapidly and to what products? What is its stability on storage to heat, light, water, metals, etc.?
- Is it impact sensitive?
- Is it toxic? If so, what is the type of hazard (inhalation, ingestion, skin contact)? What protective measures are required?
- What is the recommended first aid treatment in case of an accidental exposure?
About the reaction itself, ask:
- How violent will it be?
- What is the effect of catalysts or inhibitors?
- Will water or air affect the reaction?
What would happen and what should be done if:
- Electric power fails?
- Cooling system fails?
- Pressure gets out of hand?
- Water leaks into system?
- Reaction container falls and breaks or spills contents?
Remember that many explosions, fires, and asphyxiations are caused by the accidental combination of potentially dangerous substances.
Carrying Out Experiments
- Avoid working alone, especially when carrying out a new or unfamiliar reaction or operation.
- Make arrangements with other persons in the building to check with each other periodically.
- Never perform hazardous operations while alone.
- Use a towel to protect the hands when glass rods, tubing, or thermometers are being inserted or removed from rubber stoppers or when trying to loosen ground glass joints that are stuck.
- Do not use flawed glassware. Chipped or cracked glassware can produce serious cuts, explosions, or bad spills.
- All electrical equipment should be properly grounded. Use of extension cords should be avoided.
- In working with fume hoods the following facts should be kept in mind.
- Lowering the sash will increase air velocity and offer greater protection from toxic fumes and will provide supplemental eye and face protection. Normally the sash opening should be less than 18". If the sash is opened to a height greater than 18", an alarm is activated. It is important to keep the sash below the 18" mark. The OESO periodically measures the airflow in the fume hoods. They have labeled the hoods the maximum recommend sash height. If the sash is raised above the indicated height, then the airflow will fall below the minimum acceptable level of 75 feet per minute. If you have questions about the airflow in your fume hood, contact the OESO at 684-2794.
- Placing equipment deep in the hood will also reduce the possibility of fumes escaping into the laboratory.
Personal Safety Considerations
- Gloves (see Personal Protection chapter) should be worn when handling:
- Most kinds of organic chemicals. If in doubt, assume they are carcinogenic, toxic or allergenic.
- Corrosive materials.
- Radioactive materials.
- Pathological microorganisms.
- Large quantities of volatile solvents.
- Any highly poisonous substance.
- A lab coat or apron should be worn when working with hazardous materials.
- Use a protective shield for unfamiliar or potentially hazardous reactions.
- Wash hands often — always before eating, smoking, or leaving the laboratory. Washing should be an instinctive reaction to spillage of any chemical on the skin.
Reactions involving solutions
- No solutions should be pipetted by mouth. An aspirator bulb or vacuum line should be used.
- Add the more concentrated solution to the less concentrated — e.g. concentrated acid to water, not water to acid.
- Never mix concentrated solutions of highly reactive materials together without careful planning.
- Avoid accidentally mixing incompatible chemicals. Since both HNO3 and alcohol are often used for cleaning, it is easy to inadvertently make an explosive mixture approaching nitroglycerine in sensitivity and destructive power!
- Do not add a solid to a liquid near its boiling point. Bubble nucleation can produce a vigorous eruption.
- Avoid overheating oil baths — these can spatter and flash into a flame. Never use an oil bath for heating highly oxidizing substances. (perchlorates, nitrates, peroxides).
- Do not overfill reaction vessels — leave at least 20% free volume.
- Place a container under a reaction vessel to contain the spillage should the vessel break.
- Do not leave syringes with needles in positions where the needle is exposed to a working area. Needle tips on loaded syringes should be covered with a small cork or rubber septum when not actually in use. Accidental injection of most chemicals into a person will probably cause death.
Distillations
- When possible, the heat source should be elevated on a jack or removable blocks.
- Do not open a system to the air until the residue has cooled to avoid exothermic decomposition.
Vacuum Operations
- Vacuum desiccators and Dewar flask should be enclosed or wrapped with tape to prevent flying glass in case of an implosion. Never carry an evacuated desiccator!
- Do not apply a vacuum to a flat-bottomed flask.
- Maintain a cold trap between a vacuum pump and the apparatus — do not use liquid N2 as trap coolant when pumping organic compounds (liquid O2 may condense in the trap, leading to explosive oxidation).
- All workers utilizing the house vacuum line must be sure to use a trap to prevent volatile materials from going into the vacuum line. If the trap is not used, a dangerous amount of the volatile materials will collect along the vacuum line and in the mechanical area of the basement causing a serious fire hazard as well as a hazard to those who work in the basement.
Disposal of Waste Chemicals
Information regarding Duke University's Chemical Waste Policy is available from the OESO. Subjects covered below are specifically applicable to disposal of waste chemicals in the Department of Chemistry.
Hazard Categories
Chemicals of different hazard groups require different disposal methods. Therefore every effort must be made by the researcher to help identify the hazards associated with a chemical prior to disposal. Hazard categories of interest are as follows:
Flammables / Ignitables
Any liquid, solid, or gaseous material with a flash point less than 60 °C (140 °F) or, when ignited, burns so vigorously that it creates a hazard. Also included under this heading are any strong oxidizing materials, organic solvents (ether, xylene, toluene, etc.), alcohols (methanol, ethanol, butanol, etc.), paint thinners, and many cleaning compounds. Oxidizers, such as hydrogen peroxide and benzoyl peroxide, may cause a material to ignite spontaneously and are therefore included in this class.
Corrosives
Any aqueous solution with a pH less than 2.5 or greater than 12.5 is a corrosive hazardous waste. Strong acids (hydrochloric acid, sulfuric acid, etc.), strong bases (sodium hydroxide, potassium hydroxide, etc.), and any other materials which will cause steel to rust significantly are also considered to be in this group.
Toxics
Most pesticides and any of the 750 additional compounds named in 40 CFR 261.33 (listed in the Duke University Chemical Waste Policy) are considered to be toxic. Among the compounds named in 40 CFR 261.33 are the known carcinogens, mutagens, and teratogens. Potassium dichromate, pesticides (enrin, lindane, etc.), and silver nitrate are some examples of this group.
Reactives
Any material which is unstable, reacts violently with water, forms explosive mixtures with water, is a cyanide or sulfide bearing waste, or is capable of detonation either deliberately or inadvertently, such as sodium metal, potassium metal, dry picric acid, sodium azide, or peracetic acid, is a reactive hazardous waste. Do not place explosive chemicals into a package for pickup until receiving instructions from the OESO. If you have any questions concerning your waste category, contact Ms. Kathleen Ingram at the OESO, 684-2794.
Metals
Separate Waste containing arsenic, barium, cadmium, chromium, lead, mercury, selenium or silver. List the amount or % of each metal present in the waste container.
Radioisotopes
Separate radioisotope waste. List the specific isotope(s), the amount of each isotope and the medium the isotope is in.
Hazardous Waste Disposal
In order to dispose of chemical or radioactive waste your lab must be registered with the Environmental Programs Division of the OESO. Upon registration your lab is issued a generator ID and barcode labels which you will need for waste disposal procedures. If you do not know the generator ID for your lab contact the OESO directly. Contact the OESO (684-2794) if you need additional barcode labels for your laboratory. Information on all types of waste is maintained on the OESO website under the "Environmental Programs" section. The OESO maintains a list of chemicals that are considered to be hazardous (called the P waste list), policy and procedure , and a waste log. Be sure to follow procedures outlined by the policies. The OESO expects each lab to put forth their best effort in minimizing waste generation in the lab. To assist in the effort the OESO operates a chemical exchange program to provide, free of charge, unused chemicals to laboratories at Duke. The inventory for the exchange is created from unopened chemicals received as waste from the University and Medical Center. Call the OESO for more information about inventory and procedures for obtaining chemicals from the exchange.
Halogenated and Non-Halogenated Waste Solvents
Whenever possible, halogenated and non-halogenated solvents should be placed in separate containers for waste disposal. This is because the disposal cost for halogenated materials is significantly higher than that for non-halogenated materials. Flame resistant, five gallon, red cans can be obtained from the OESO for this purpose. These cans are to be used only for solvents and should never be used for disposing of any heavy metals, reactives, acids, bases or bromine. Cans are marked with either yellow (for non-halogenated solvents) or green (for halogenated solvents) tape. In addition, each can has an associated (yellow or green) inventory sheet with a place for a single barcode label and room to indicate the volume and name of each waste solvent added to the can. Each laboratory can have a maximum of four red cans for disposal of solvents. Solvent cans are picked up by OESO personnel. To let the OESO know that your solvent cans are full, complete a disposal request through the Waste Pickup Request System. Cans must be barcoded and paperwork filled out to the best of your ability, otherwise your solvent can will not be picked up. Due to federal and state regulations governing disposal, it is vitally important that the contents of each red can are accurately reflected on the paperwork. Links to logsheets, waste accumulation labels, small container labels, the waste pickup request system, etc are located on the OESO website: Environmental Programs Division, Chemical Waste Section.
Request for Pickup of Other Chemical Waste
To dispose of chemical waste not collected in the red cans, each item will need to be barcoded, inventoried and submitted for waste disposal through the Waste Pickup Request System. You will need to list the proper name of the chemical (abbreviations and chemical structures are not acceptable) and the size of the container on the pickup request form. Once your request is received the OESO will process the request and pickup your waste. In the event that your request is not properly completed, or there are chemicals on the request form which are not already in the OESO database, pickup will be delayed. Under normal conditions your waste should be picked up within 5-10 days after its submission. Once you have submitted a request for pickup, keep the waste in an easily identified area and let at least one other lab member know that you are expecting a pickup and the location of the waste materials. Be sure, also, that the waste is not in a leaking, or improperly sealed container. If there are any problems with the condition or containment of the material you will have to resolve them before pickup is completed.
Special Notes
Waste containing arsenic, barium, cadmium, chromium, lead, mercury, selenium or silver are particularly difficult and expensive to dispose of. Consequently, specific information giving the amounts and types of metals present the in the chemical waste must be included on the chemical pickup request form. If the information is not provided on the form, the OESO will contact your lab prior to pickup to obtain the missing information. If the exact amount of a metal in the waste is not known a good estimate should be made based on how much material was used in the experiment generating the waste. Mercuric cyanide does not currently have a disposal route in the United States. Labs are strongly encouraged to find a substitute for this chemical. In the event that your lab must use mercuric cyanide, please get approval from the OESO prior to purchasing the material.
Disposal of Empty Disposable Gas Cylinders
The contents of lecture bottles must be identified prior to disposal with the OESO. Every effort should be made to recycle a used, but not empty, lecture bottle by giving it to another researcher when your lab no longer needs the material.
- Open valve fully so that the top of the container may be completely unscrewed or sawed off prior to burial in a landfill. Then tag the cylinder for waste pickup.
- If the tank has an automatic valve that cannot be left open, (e.g., disposable propane tanks for torches) simply tag it for pickup when empty.
- Do not vent toxic or other unsafe gasses in the fume hood. Instead, you should attempt to cycle the bottle to another researcher, or if it clearly needs to be emptied, you should trap the gas in an appropriate solution for disposal.
Disposal of Used Silica Gel
Used silica may be left in a hood, to evaporate solvent, packaged in thick plastic bags or sealed drums and disposed of in the dumpster at the loading dock provided that it does not contain other hazardous chemicals. If it does contain hazardous chemicals dispose following guidelines for chemical waste. Do not dispose of silica gel in trash cans or leave it for housekeeping to remove.
Disposal of Rinse Solvents
When you rinse glassware with a solvent, such as acetone, collect the rinse solvent in an appropriate container. Preferably, rinse your glassware over a funnel that empties into a container such as a bottle or used acetone can. The container should then be tagged for waste pickup using normal procedures. Our general policy is that nothing except water should go down the drain.
Disposal of Empty Bottles and Cans
Housekeeping will collect empty bottles and cans left in the main hallway. To avoid exposing housekeeping staff to chemicals, any bottles or cans that you place in the hallway for disposal must be thoroughly cleaned and rinsed.
Disposal of Broken Glass
Place all broken glass items in the cardboard containers designated for the disposal of broken glass. The box should be properly labeled, and taped shut when it is full. Housekeeping will dispose of properly labeled and taped broken glassware boxes.
Disposal of Hypodermic Syringes and Needles
Used needles cannot be placed intact in regular waste receptacles since they may injure persons who must handle our trash. Use only containers specifically designated for disposal of syringes and needles. These containers are available upon request from OESO. Full containers of used syringes and needles should be tagged as chemical waste as described above.
Disposal of Used Pump Oil
Used pump oil should be tagged as chemical waste as described above.
Disposal of Smelly Trash
In order to avoid exposing Housekeeping staff to unusually odoriferous trash, bag and tie the trash before putting it in waste baskets.
Disposal of Chemicals in the Trash is Forbidden
If the housekeeping staff sees (or smells) chemicals in the trash, they will not pick it up. The Administrative Coordinator will be notified, and the lab occupants will be contacted to correct the problem.
Serious emergencies are of two basic types — those that expose one or two individuals to personal injury (burn, shock, poisoning, etc.) and those that endanger lives and property in the whole building. In either sort of emergency situation you should get help at once. This may be done by:
- calling for help from people nearby, and/or
- calling the University Public Safety Office at 911 from a CAMPUS phone or 684-2444 from a cell phone.
If you and/or the people nearby successfully handle the emergency without calling Campus Police, you still need to report the emergency within 24 hours to the Departmental Safety Coordinator and to Campus Police at 684-2444 (the 911 number should not be used for this type of report).
Personal Injury
Except for trivial accidents, call the University Public Safety Office by dialing 911 from a CAMPUS phone or 684-2444 from a CELL phone. State clearly:
- What the emergency is
- Where the emergency is
- Who is calling
- Where to pick up victim
- Do not hang up the phone until the dispatcher hangs up.
The car from the Public Safety Office should arrive within 5 minutes.
First Aid
Give First Aid only if there is an immediate life threatening accident or if the accident is trivial; otherwise await the arrival of qualified safety personnel or paramedics. If you believe that you must provide first aid, here are some suggestions.
- Thermal Burns. In the case of a burn, apply cold water and/or ice immediately to the burned area until the pain subsides. Wrap the burned area to protect from infection. It is best to avoid oils and ointments in first aid treatment since these frequently complicate the physician's job. If the burns are extensive, treat for shock (see following) and call Public Safety (911 from a CAMPUS phone or 684-2444 from a cell phone) for transportation.
- Chemical Burns. Flush the affected area at least 15 minutes with tap water. Acid or minor bromine burns may then be treated with 5% sodium carbonate solution. Alkali burns can be washed with 5% acetic acid solution or saturated boric acid solution. Wrap the burned area loosely. Call 911 from a CAMPUS phone or 684-2444 from a cell phone for severe burns.
- Burns from the following chemicals. Hydrofluoric acid, white phosphorus and phenol require special treatment. If these chemicals are to be handled, be sure that you know the first aid treatment before they are used. See their MSDS sheets.
- Minor Bleeding. Allow the blood to flow a few moments. Flush the wound thoroughly with water. Apply an antiseptic and bandage the wound to prevent contamination.
- Toxic Fumes. If there are complaints of a headache or dizziness in a laboratory in which the odors of such toxic gases as chlorine, hydrogen sulfide, nitrogen oxides, etc., are even detectable, see that the victim is taken to fresh air immediately and made to rest. Do not permit the victim to move unless the symptoms disappear rapidly. Also clear all others from the laboratory until the problem can be clearly identified and remedied. Call 911 from a CAMPUS phone or 684-2444 from a cell phone.
- Fainting and Shock. Any or all of the following are symptoms of shock: chill, trembling, shortness of breath, pallor, nausea, excessive perspiration. In such cases, the patient should be placed in a prone position with head lower than feet. Loosen tight clothing and keep patient warm. Call 911 from a CAMPUS phone or 684-2444 from a CELL phone and request transportation.
- Chemical Splashes. Safety Showers and Eye Washes are located in each of the laboratories. Since it has been conclusively proven that immediate washing of the skin with a generous amount of water is the most effective first aid treatment for chemical burns. Pulling the ring hanging from a safety shower will cause the rapid discharge of water. Be sure to leave the ring hanging freely at the end of the chain at all times. For a major chemical splash or clothing on fire, have no hesitation to use the shower in your laboratory. For less serious splashes requiring change of clothes, use the showers located in the restrooms. All of the research and teaching laboratories are also equipped with eye wash fountains in case a chemical splashes in the eyes.
Proper documentation and follow-up are the keys to preventing recurrence of any accident. The Incident Report Form (below) should be filled out in the event of any personal injury, fire or chemical spill. If an injury occurs to an employee or teaching assistant who requires medical attention, then a Work-Related Injury or Illness form must be completed within 24 hours online via the Duke Human Resources website. The form requires you to enter your immediate supervisor's name. For personnel working in research labs, the immediate supervisor is your PI.
Emergencies Requiring Outside Professionals (Police, Fire Department)
If the emergency endangers lives and property in the building, you or someone helping you will need to pull the red alarm levers which will call the University Public Safety Officers and which is the signal for evacuating the building. (See section on evacuation for details of evacuation plan for the building.) The alarm pull stations are located near exit doors. Call, or have someone call, Duke Public Safety at 911 from a CAMPUS phone or 684-2444 from a CELL phone and give the following information concerning the emergency:
- Location - Room _____, French Family Science Center. What you see: Flame or smoke. Flame and smoke. Describe smell of smoke. Describe odor of gas. Serious injuries involved. What is burning or what was spilled. Flammable liquids (kind), combustibles (kind), toxic substance, etc. Quantity in room, if known. Name of caller.Phone number where you can be reached. Stay on the phone until the dispatcher hangs up.
- You or people helping you should be sure that:In an emergency situation the hierarchy of responsibility for determining who is in charge until the professional safety personnel arrive is as follows:As soon as professional safety personnel arrive, they have the responsibility and authority to take charge of the situation, although they may still need the help of Chemistry Department personnel. In a case of more serious accidents where there is injury and the possibility of a fire or explosion, the City Public Safety Officers (firemen and police) may be called to the French Family Science Center. When they are here, they have the responsibility of assuming as much authority for what is done as they consider appropriate. This means that they can call for evacuation of the building, and if they do, their orders are to be complied with at once without question. Failure to do so or to offer resistance to the orders of a Public Safety Officer can result in arrest. It is important to understand that Public Safety Officers are not chemists and may not be able to size up or appreciate the difference between small and large hazards as they would appear to us. They do, however, have the legally constituted responsibility and authority to deal with potentially hazardous situations and often must start by assuming a "worst case scenario" although the real hazard may quickly turn out to be small. Since all research personnel have been trained to respond to building evacuation orders there should be little problem in closing down an experiment for 10 to 15 minutes until the responsible Public Safety Officer has been satisfied that the building may be reoccupied safely.
- emergency personnel from outside the French Family Science Center have been called,
- initial steps have been taken to deal with the situation, such as administering first aid (if qualified), containing or extinguishing a fire or closing all fire doors if you are unable to fight the fire, cleaning up a chemical spill, etc.,
- information on the hazards of the situation or injured person will be given to professional safety personnel when they arrive,
- the appropriate research director(s) and officers of the department have been informed of the emergency situation.
- faculty supervisor of the group having the accident,
- the postdoctoral or graduate student Safety Officer for the laboratory or research group involved with the emergency,
- any other member of the group having the accident or any faculty member.
Emergency Evacuation
In the event of a serious emergency such as fire, explosion or release of hazardous gas, the building must be evacuated. This requirement is not only to remove those present in the building from potential hazard, but to clear the way for firemen or Safety Officers who are responsible for handling the emergency. The FOUR emergency access routes that may be used by emergency responders coming to the French Family Science Center are the front of the building (facing Research Drive), the LaSalle Street entrance in front of Phytotron, the Chemistry Parking lot entrance between Gross and FFSC, and the entrance behind the Physics Building.
If an evacuation alarm is sounded, before leaving a lab, you should shut down all experiments as quickly and safely as possible using common sense as your guide. This should include removing heat, releasing pressure or vacuum, and turning off electrical appliances. When you exit from a lab; leave the lights turned on, close the doors to the room, but leave the doors unlocked. Follow the designated evacuation route from your lab using the stairs, not the elevator. Move to a designated safe area that has been pre-arranged for your lab and meet with the other members of your research group to verify that everyone has left the building. Inform emergency responders if a member of your group remains in/did not make it out of the building.
Electrical exit signs have been installed throughout the building. Follow the overhead exit signs or take the nearest stairway down and exit from the building.
- If you are on an upper floor in the south end (the Gross Chemistry end) of the French Family Science Center, take stairs down to level-1, exit and go to your right on to the grassy area near the greenhouses located behind the French Family Science Center. Designated as "Safe area #2" marked in green on the diagram below. Remain in the grassy area until instructed by authorities to move further from the building or to return to the building.
- If you are located on an upper floor near the middle of the building, take stairs down to the main lobby (level-2) and exit by the front doors out to the grassy terraced area in front of the French Family Science Center. Designated as "Safe area #1" marked in green on the diagram below. Remain in the terraced area until instructed by authorities to return to the building.
- If you are on an upper floor in the north end (the Physics end) of the French Family Science Center, take stairs down to level-2, exit and go to your left to the raised concrete parking area behind the Physics building. Designated as "Safe area #3" marked in green on the diagram below. Remain in the parking area until instructed by authorities to move further from the building or to return to the building.
- Teaching assistants with laboratory classes on level-1 should tell their students to check their experiments carefully before leaving to see that everything is turned off and safe until they can return to the laboratory and then evacuate and the building immediately. The class should be told to follow the evacuation route indicated by electrical exit signs and to gather together as a group in the grassy area near the greenhouses located behind French Family Science Center, designated as "Safe area #2" marked in green on the diagram below, OR near the statue of the camel, designated as "Safe area #4. Remain in these grassy areas until instructed by authorities to move further from the building or to return to the building.
- If you are otherwise on level-1, follow the overhead electrical exit signs that will take you to the nearest exit that takes you onto the grassy area near the greenhouses located behind the French Family Science Center. Designated as "Safe area #2" marked in green on the diagram below. Remain in the grassy area until instructed by authorities to move further from the building or to return to the building.
During fire drills a verbal all-clear signal will be given by an authority when you may return to the building. Do not return to the building until the all-clear signal is given. Evacuation drills will be held twice per year as required by the City of Durham Fire Marshall. Total evacuation of the building is required during evacuation drills. In addition to the drills, the fire alarm system is tested once per year. It should be recognized that any building evacuation, either in a drill or in a genuine emergency situation, may interfere with experiments that are underway. You should keep this possibility in mind and consider as part of your preparation for any experiment the question of how you can safely close it down in an emergency with minimum damage to your work.
Procedures in Case of Electrical Power Failure of Loss of Water Pressure
If the power fails, a backup generator will come on within about 30 seconds. Notify Maintenance immediately of a power outage or loss of water pressure by calling 684-2122, 8 AM to 5 PM, Monday through Friday. At other times, try the same number first, but call Public Safety at 684-2444 if you get a recorded message indicating that no maintenance workers are on duty.
Department Safety Forms
Easily Printable Safety Forms (PDF format):
Lab Safety Links
Here are a collection of safety related links. If you come across any other good sites, please e-mail the links to webmaster@chem.duke.edu.
Duke University Safety Sites
- Duke University Safety Manual
- OESO Safety
- OESO Biological Safety
- Duke University's Particularly Hazardous Substances Index
- Duke University Surplus Chemical Exchange
- OESO Laboratory Safety
- OESO Environmental Programs
- OESO Fire Safety
- OESO Radiation Safety
- Duke University / DUMC Laser Safety Policy
Below is a list of links providing safety information. This information comes from the American Chemical Society and was published in CHEMTECH, November 1999, page 56.
SDS Information
- SDS searchable database
- SDS searchable database
- SDS searchable database
- OSHA's Hazardous Communications Regulations
Agency of Toxic Substances and Disease Registry
- Agency of Toxic Substances and Disease (ATSDR)
- ATSDR Health Advisory
- ATSDR Alerts
- ATSDR Frequently Asked Questions about Toxic Substances
- Public Health Assessments searchable database
Food and Drug Administration
Environmental Protection Agency
- Environmental Protection Agency (EPA)
- EPA Office of Air and Radiation (OAR)
- OAR description of toxic air pollutants
- EPA Indoor Air Quality (IAQ)
- List of IAQ publications
- EPA Office of Water Quality (OW)
- Office of Water's current drinking water standards
- Office of Water's beach watch
- Office of Wastewater Management (OWM)
- EPA Pesticides division
- Office of Pollution Prevention and Toxics(OPPTS) concerns for citizens site
- Solid waste and emergency response site
- Office of Solid Waste information resources
- Information on chemical emergency preparedness
Chemical Hygiene
- Duke OESO, Chemical Hygiene Information
- Chemical Waste Management Guide
- Chemical health and safety data information
- Chemical and Engineering news safety letters
The Right Glove for the Job
The glove charts for the department of chemistry are organized in three ways. (See following Chapter of Safety Manual.) First, the physical characteristics of glove material is charted. In the second chart, glove material degradation in specific compounds is examined. Finally, the permeability of compounds in gloves is analyzed in chart three.
For more information check out the glove compatibility guide at http://www.ehs.ufl.edu/Lab/CHP/gloves.htm or call the Environmental Safety Office at 684-2794.
Online Safety Training (OESO)
Required for anyone who will be working in a research lab
In order to have any building key issued, all three of the following online safety training courses must be completed, which includes taking the quiz at the end of each training course. Print out the pages that state you have passed each of the three training quizzes and bring them with you when having your safety release form signed and keys issued. The safety training modules may be accessed by visiting the Duke Occupational & Environmental Safety Office site. Click on "Training & Reports" and "Online Training". You will log in using your NetID and password.
- Hazard Communication for Lab Personnel
- Fire/Life Safety Training
- Laboratory Safety – General
Required for a staff member who will only be working in an office
In order to have any building key issued, the following online safety training courses must be completed, which includes taking the quiz at the end of each training course. Print out the pages that state you have passed the training quizzes and bring them with you to get keys issued. The safety training modules may be accessed by visiting the Duke Occupational & Environmental Safety Office site. Click on "Training & Reports" and "Online Training". You will log in using your NetID and password.
- Ergonomics Overview
- Fire/Life Safety Training
Physical Characteristics of Glove Materials*
| Material (Designation in Matrices) | Abrasion Resistance | Cut Resistance | Flexibility | Heat Resistance |
|---|---|---|---|---|
| Chlorinated Polyethylene (CPE) | E | G | G | G |
| Material (Designation in Matrices) | Ozone Resistance | Puncture Resistance | Tear Resistance | Relative Cost |
|---|---|---|---|---|
| Chlorinated Polyethylene (CPE) | E | G | G | Low |
Degradation
Degradation is a reduction in one or more physical properties of a glove due to chemical contact. Exposed gloves may swell, get harder or softer, stiffen or weaken or become brittle. Degradation-resistance testing of gloves is important to assure worker safety, however, permeation-resistance is also essential.
Data shown are No performance warranty in intended or implied. Ratings were arrived at by visual and physical examination of samples after their exposure to the chemical stated. Sources for this information include studies conducted at the University of Washington and Prudent Practices for Handling Hazardous Chemicals in Laboratories, 1981.
When considering a glove for a specific application, it is important to consider other requirements such as thermal conditions, chemical concentration, physical hazards and mixtures of chemicals. A glove should be tested in actual service before specification.
| NEO SUP | Neoprene Supported | NAT SUP | Natural Rubber Supported | |
| NEO UNSUP | Neoprene Unsupported | NAT UNSUP | Natural Rubber Unsupported | |
| NIT SUP | Nitrile Supported | PVC SUP | PVC Supported | |
| NIT UNSUP | Nitrile Unsupported |
| E | Chemical has little or effect | G | Chemical has minor effect | |
| F | Chemical has minor to moderate effect | P | Chemical has moderate to severe effect | |
| NIT SUP | Nitrile Supported | PVC SUP | PVC Supported | |
| NR | Chemical has severe effect | NT | Chemical not tested |
| Chemical | Cas # | NEO SUP | NEO UNSUP | NIT SUP | NIT UNSUP | NAT SUP | NAT UNSUP | PVC SUP |
|---|---|---|---|---|---|---|---|---|
| Acetaldehyde | 00075070 | F | F | F | F | P | P | P |
| Acetic Acid (Glacial) | 00064197 | E | F | E | E | E | G | E |
| Acetic Acid 50% | 00064197 | E | G | E | E | E | G | E |
| Acetone | 00067641 | E | E | F | F | E | F | E |
| Acetonitrile | 00075058 | G | F | P | P | P | P | NR |
| Acrylonitrile | 00107131 | G | G | G | G | P | P | F |
| Ammonium Hydroxide 30% | 01336216 | E | E | E | E | E | E | E |
| Amyl Alcohol | 00071410 | E | E | E | E | G | G | E |
| Aniline | 00062533 | P | NR | P | P | NR | NR | NR |
| Animal Fats | E | E | E | E | F | F | G | |
| Benzaldehyde | 00100527 | P | P | P | P | P | P | P |
| Benzene | 00071432 | NR | NR | NR | NR | NR | NR | NR |
| Benzyl Alcohol | 00100516 | G | G | G | G | F | F | G |
| Benzyl Chloride | 00100447 | P | P | P | P | NR | NR | P |
| Borax (Saturated) | 01303964 | E | E | E | E | G | G | G |
| Bromine | 07726956 | G | G | NT | NT | G | G | G |
| Butane | 00106978 | E | E | NT | NT | P | P | P |
| Butraldehyde | 00123728 | G | G | NT | NT | P | P | G |
| Butyl Acetate | 00123864 | F | F | F | F | P | P | P |
| Calcium Chloride | 10035048 | E | E | E | E | E | E | E |
| Calcium Hypochlorite | 07778543 | G | G | G | G | G | G | G |
| Calcium Hydroxide | 01305620 | E | E | E | E | E | E | E |
| Carbon Disulfide | 00075150 | P | P | G | G | P | P | F |
| Capryl Alcohol | 00123966 | G | G | G | G | F | F | G |
| Cellosolve Acetate | 00111159 | F | P | F | F | P | P | F |
| Chlorine (gas) | 07782505 | E | NT | E | NT | NT | NT | F |
| Chloroacetone | 00078955 | E | E | NT | NT | F | F | P |
| Chlorobenzene | 00108907 | P | NR | P | P | NR | NR | P |
| Chloroform | 00067663 | F | P | F | F | NR | NR | F |
| Chromic Acid | 01333820 | F | F | F | F | P | P | E |
| Citric Acid | 00077929 | E | E | E | E | G | G | G |
| Coconut Oil | E | E | E | E | P | P | G | |
| Corn Oil | E | E | E | E | P | P | G | |
| Cresols | 01319773 | F | P | F | F | NR | NR | F |
| Cutting Oil 10% | G | G | G | G | P | P | G | |
| Cyclohexane | 00110827 | E | E | NT | NT | F | F | P |
| Dibenzyl Ether | 00103504 | G | G | NT | NT | F | F | P |
| Dibutylphthalate | 00084742 | E | E | E | E | F | F | P |
| Diethanolamine | 00111422 | E | E | NT | NT | F | F | E |
| Diethylamine | 00109897 | F | P | F | F | P | P | P |
| Diesel Fuel | E | E | E | E | P | P | G | |
| Diethyl Ether | 00060297 | F | P | F | F | P | P | P |
| Dimethylformamide | 00068122 | G | F | G | G | P | P | P |
| Dimethylsulfoxide | 00067685 | E | G | E | E | P | P | F |
| Dioctyl Phthalate | 00117840 | E | G | E | E | F | F | P |
| Dioxane | 00123911 | F | F | F | F | P | P | F |
| Ethanol | 00064175 | E | E | E | E | E | E | E |
| Ethyl Acetate | 00141786 | G | F | F | F | F | F | P |
| Ethyl Benzene | 00100414 | P | NR | P | P | NR | NR | P |
| Ethyl Ether | 00060297 | F | P | F | F | P | P | P |
| Ethylene Dichloride | 00107062 | F | F | G | G | P | P | P |
| Ethylene Glycol | 00107211 | G | F | G | G | F | F | G |
| Formaldehyde 37% | 00500000 | G | E | E | E | E | E | E |
| Formic Acid | 00064186 | G | G | F | F | G | G | G |
| Gasoline | 08006619 | E | G | E | E | P | P | F |
| Glycerine | 00056815 | E | E | E | E | G | G | E |
| Glycine | 00056406 | E | E | E | E | G | G | E |
| N-Heptane | 00142825 | E | G | E | E | P | P | G |
| N-Hexane | 00110543 | E | G | E | E | P | P | F |
| Hydraulic Fluid | E | G | E | E | P | P | G | |
| Hydrobromic Acid 40% | 10035106 | E | E | NT | NT | G | G | E |
| Hydrochloric Acid 10% | 07647010 | E | G | E | E | E | G | E |
| Hydrochloric Acid 36% | 07647010 | F | P | F | F | P | P | P |
| Hydrofluoric Acid 30% | 07664393 | G | G | G | G | G | G | E |
| Hydrogen Peroxide | 07722841 | G | G | G | G | G | G | E |
| Isooctane | 01111659 | E | G | E | E | P | P | F |
| Isopropyl Alcohol | 00067630 | G | G | G | G | G | G | G |
| Kerosene | 08008206 | E | G | E | E | P | P | G |
| KOH 45% | 01310583 | E | E | E | E | G | G | G |
| Lactic Acid 85% | 00050215 | G | G | G | G | G | G | G |
| Lauric Acid | 00143077 | G | F | G | G | P | P | F |
| Ligroine | 08032324 | E | G | E | E | P | P | G |
| Linseed Oil | G | G | G | G | P | P | F | |
| Maleic Acid | 00110167 | G | G | G | G | F | F | G |
| Methylamine | 00074895 | F | P | F | F | P | P | P |
| Methanol | 00067561 | G | G | G | G | G | G | F |
| Methyl Ethyl Ketone | 00078933 | P | P | NR | NR | F | F | NR |
| Methylene Chloride | 00075092 | NR | NR | NR | NR | NR | NR | NR |
| Methyl Isobutyl Ketone | 00108101 | P | P | NR | NR | P | P | NR |
| Mineral Oil | 08012951 | E | G | E | E | P | F | E |
| Mineral Spirits | E | G | E | E | F | P | E | |
| Morpholine | 00110918 | E | E | NT | NT | F | F | E |
| Monoethanolamine | 00141435 | E | E | NT | NT | F | F | E |
| Nitric Acid 10% | 07697372 | G | F | G | G | P | P | F |
| Nitric Acid 30% | 07697372 | P | P | P | P | NR | NR | NR |
| Nitrobenzene | 00098953 | P | P | P | P | NR | NR | P |
| Octane | 00111659 | E | G | E | E | P | P | F |
| Oleic Acid | 00112801 | G | F | G | G | P | P | F |
| Olive Oil | 08001250 | E | G | E | E | P | P | F |
| Oxalic Acid | 00144627 | G | G | G | G | G | G | G |
| N-Pentanol | 00071410 | E | E | E | E | G | G | G |
| Perchloric Acid | 07601903 | G | G | F | F | F | F | E |
| Phenol | 00108952 | E | E | NT | NT | G | G | E |
| Perchloroethylene | 00127184 | F | P | F | G | NR | NR | P |
| Phosphoric Acid | 07664328 | E | E | NT | NT | G | G | E |
| Pine Oil | 08001093 | G | G | G | G | P | P | G |
| Potassium Hydroxide | 01310583 | E | E | E | E | E | E | E |
| Potassium Permanganate 10% | 07711647 | G | F | G | G | P | P | F |
| Sodium Hydroxide 50% | 01310732 | E | E | E | E | E | E | E |
| Sodium Hypochlorite | 07681529 | P | P | F | F | G | G | G |
| Stearic Acid (saturated) | 00571114 | E | G | E | E | G | G | G |
| Sulfuric Acid 10% | 07664939 | G | G | G | G | G | G | G |
| Sulfuric Acid 20% | 07664939 | F | F | F | F | F | F | F |
| Toluene | 00108883 | F | P | F | G | NR | NR | F |
| 1,1,1-Trichloroethane | 00071556 | F | P | F | G | NR | NR | P |
| Trichloroethylene | 00079016 | F | P | F | G | NR | NR | P |
| Tricresyl Phosphate | 01330785 | F | F | NT | NT | P | P | F |
| Triethanolamine | 00102716 | E | E | E | E | F | F | E |
| Vegetable Oils | E | E | E | E | P | P | E | |
| Xylene (o, m, p) | 01330207 | F | P | F | G | NR | NR | F |
Permeation
The Permeation Resistance Guide is a reference point for proper glove analysis in hazardous chemical handling. All test results were produced independently. Radian Corporation of Austin Texas (an AIHA accredited laboratory) performed the permeation testing in accordance with ASTM Standard F739-81.
Each Polymer is Ranked according to breakthrough time as a reference for future testing by the user. Breakthrough time is the elapsed time between initial contact of an aqueous solution with the exterior surface of a glove and the time at which the solution can be detected on the interior surface.
Actual performance of a glove depends upon varied factors. Work application, chemical mix, chemical contact time, and glove thickness are just a few.
| Chemical | Best | Second | Third |
|---|---|---|---|
| Acetaldehyde | Rubber/R | Rubber/S | Neoprene |
| Acetone | Rubber/R | Neoprene | |
| Acetonitrile | Nitrile/C, Neoprene | PVC | |
| Chlorine Gas | Neoprene | Rubber/R | PVC |
| Dibutylphthalate | Neoprene | Nitrile/C | |
| Diethylamine | Rubber/R | Neoprene | PVC |
| Diethyl Ether | Nitrile/C | Neoprene | |
| Dimethylformamide | Neoprene | Nitrile/C | Nitrile/U |
| Dioxane | PVC | Nitrile/C | Neoprene |
| Ethylene Dibromide | Neoprene | PVC | Nitrile/U |
| Ethoxyethanol | Neoprene | Nitrile/C | Nitrile/U |
| Ethylene Oxide | Nitrile/U | ||
| Formaldehyde 37% | Nitrile/C, PVC, Nitrile/U | ||
| N-Hexane | Neoprene | Nitrile/U | PVC |
| N-Hexane | Neoprene | Nitrile/U | PVC |