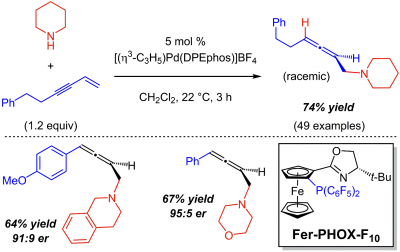
The Malcolmson lab has demonstrated that conjugated enynes undergo efficient and selective intermolecular 1,4-hydroamination to afford chiral allenes with a variety of primary and secondary aliphatic amines as well as benzophenone imine as an ammonia surrogate. A large number of allenes are obtained in racemic form with an achiral Pd(DPEPhos) catalyst. Through the design and development of a novel PHOX ligand, the group illustrates that highly enantioenriched allenes may be obtained in several cases. A perfluoroaryl phosphine was discovered to slow reaction reversibility and enable the enantioselective couplings. Read more about this methodology here in The Journal of the American Chemical Society.